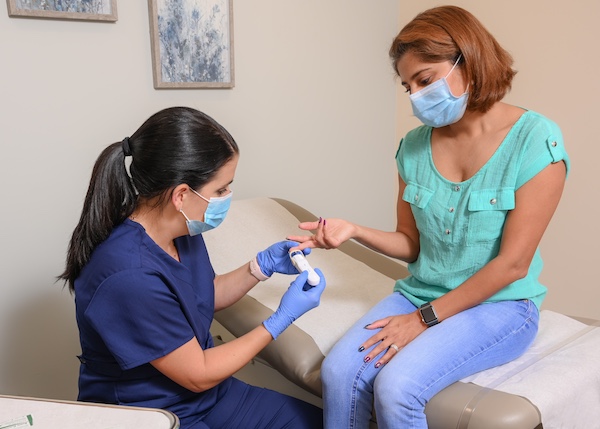
Bacterial or non-bacterial infection?
FebriDx aids in the differentiation between bacterial infection and non-bacterial etiology.
Acute respiratory infections, causing symptoms such as common cold, sore throat, cough, congestion and runny nose, are the most common reason for seeking medical advice and antibiotic prescriptions.1-3 Differentiating between bacterial infection and non-bacterial etiology can be challenging due to an overlap in signs and symptoms of bacterial and non-bacterial infections.
The majority of acute respiratory infections are caused by viruses and do not require antibiotics, yet antibiotics are prescribed in up to 50% of cases.4 Antibiotic resistance continues to remain a major global health threat contributing to 2.8 million antibiotic-resistant illnesses and 35,000 deaths each year in the U.S.5
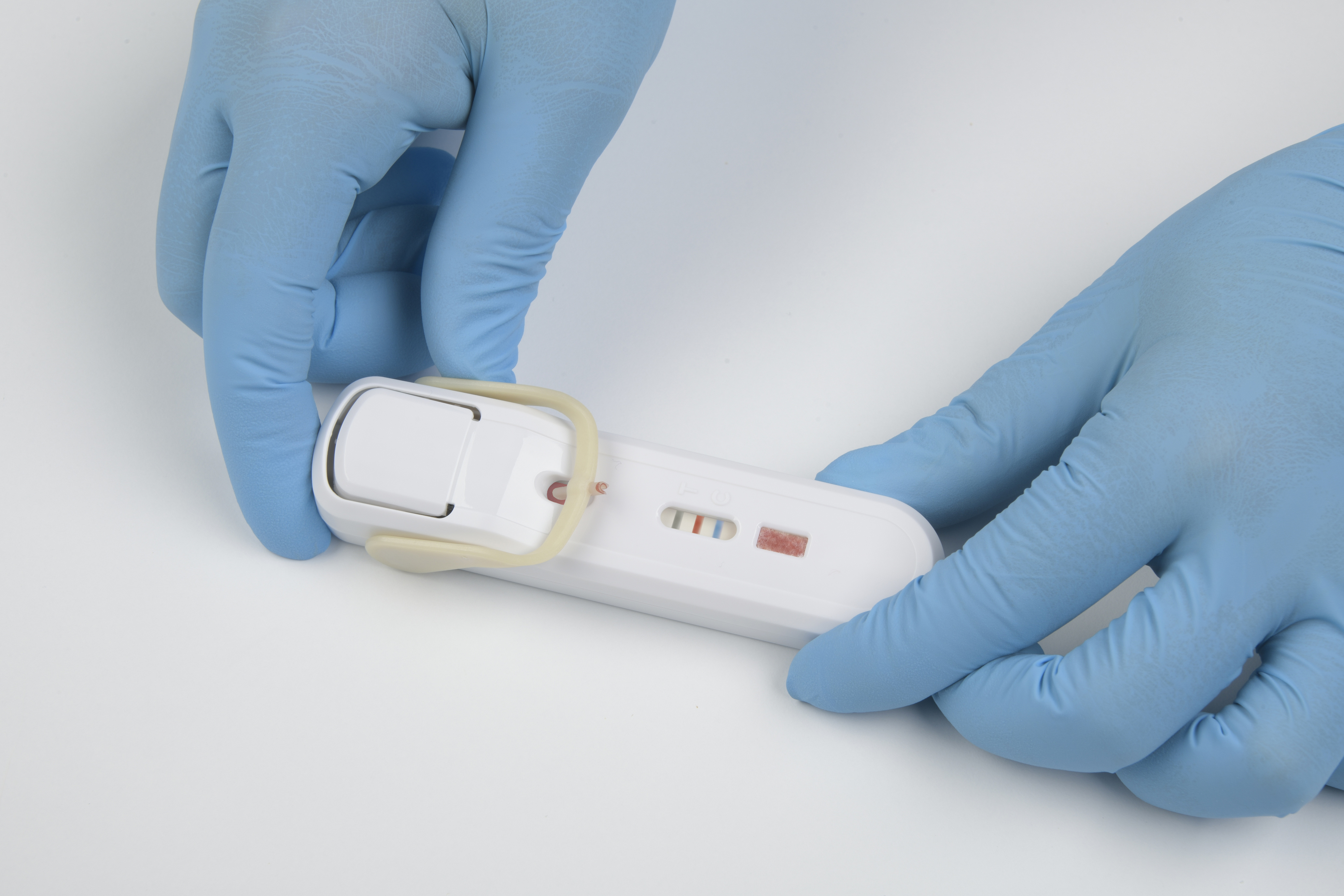
FebriDx point-of-care test device
FebriDx is a rapid point-of-care test that uses a fingerstick blood sample to aid in the differentiation between bacterial infection and non-bacterial etiology. FebriDx is intended to be used in urgent and emergency care settings.
Knowing whether a patient has a bacterial infection has a direct impact on reducing unnecessary antibiotic prescriptions, limiting the spread of antibiotic-resistant bacteria, and helping providers know when to initiate treatment.

- Results after 10 minutes increases confidence in whether or not to prescribe an antibiotic.
- Highly sensitive/specific dual biomarker technology provides reliable differentiation of bacterial and non-bacterial infections.
- All-in-one, instrument-free test device means no expensive equipment and a fully portable solution.
Acute respiratory infections create challenges.
FebriDx provides solutions.
99% NPV for ruling out bacterial infection
Using FebriDx during a patient visit can reduce diagnostic uncertainty and help guide appropriate patient management and reduce inappropriate antibiotics. Results are available after 10 minutes, allowing the clinician to provide a timely and targeted treatment plan during the initial visit.
-
Increase confidence in decision making
Confidently rule out a bacterial infection with 99% negative predictive value (NPV)6,7
-
Ensure bacterial infections are not overlooked
Increased confidence in patient diagnosis
-
Improve patient satisfaction
Support patient understanding of treatment plan with a tangible test result
-
Enable improved patient management
Actionable results after 10 minutes for use during a patient visit
-
Support antimicrobial stewardship
Reduce unnecessary antibiotic use6-8
- Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434.
- Centers for Disease Control and Prevention (CDC). National Ambulatory Medical Care Survey. National Center for Health Statistics. Updated December 30, 2021. Accessed Jan, 2024, https://www.cdc.gov/nchs/ahcd/about_ahcd.htm
- Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016;33(3):312-317.
- Centers for Disease Control and Prevention (CDC). Measuring Outpatient Antibiotic Prescribing. Updated Oct 2022. Accessed Feb 2024, https://www.cdc.gov/antibiotic-use/data/outpatient-prescribing/index.html
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2019. U.S. Department of Health and Human Services. Updated Dec 2019. Accessed Jan 2024, https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- Shapiro NI, Self WH, Rosen J, et al. A prospective, multi-centre US clinical trial to determine accuracy of FebriDx point-of-care testing for acute upper respiratory infections with and without a confirmed fever. Ann Med. Aug 2018;50(5):420-429. doi:10.1080/07853890.2018.1474002
- Shapiro NI, Filbin MR, Hou PC, et al. Diagnostic Accuracy of a Bacterial and Viral Biomarker Point-of-Care Test in the Outpatient Setting. JAMA Netw Open. Oct 3 2022;5(10):e2234588. doi:10.1001/jamanetworkopen.2022.34588
- Carlton HC, Savović J, Dawson S, Mitchelmore PJ, Elwenspoek MM. Novel point-of-care biomarker combination tests to differentiate acute bacterial from viral respiratory tract infections to guide antibiotic prescribing: A systematic review. Clinical Microbiology and Infection. 2021
FebriDx can help reduce unnecessary antibiotics and risks.
Unnecessary use of antibiotics leads to antimicrobial resistance (AMR), causing more than 1.27 MILLION DEATHS globally1
Antimicrobial resistant infections cost $BILLIONS in the U.S. each year1
Acute respiratory infections and antibiotics
Acute respiratory infections, producing symptoms such as cough, sore throat, runny nose and congestion, are the most common reason patients seek care worldwide.2-4
The majority of acute respiratory infections are caused by viruses and do not require antibiotics, yet antibiotics are prescribed in up to 50% of cases.5 The overlapping symptoms of acute respiratory infections make it challenging for clinicians to differentiate bacterial from non-bacterial infections.
Implications of unnecessary antibiotic use
There are more than 200 million antibiotic prescriptions written each year in the outpatient setting, approximately 30-50% of which are unnecessary.5 Unnecessary antibiotic use leads to increased antibiotic resistance and increased costs.6
FebriDx can help
FebriDx is intended to be used in urgent and emergency care settings. FebriDx offers rapid, point-of-care results that aid in diagnosing a bacterial infection,7,8 which could have a direct impact on the spread of resistant bacteria.
Patient satisfaction
Patients often insist on antibiotics. Since most coughs, colds, sore throats, and runny noses are caused by non-bacterial infections, antibiotics can often be prescribed unnecessarily. FebriDx produces a tangible test result that can be shared with the patient, thereby improving patient satisfaction and confidence in their treatment recommendations.
With a 99% Negative Predictive Value,7,8 FebriDx can help rule out bacterial infection and prevent inappropriate antibiotic prescriptions.
- Centers for Disease Control and Prevention (CDC). National Infection & Death Estimates for Antimicrobial Resistance. Updated Dec 2021. Accessed Jan 2024, https://www.cdc.gov/drugresistance/national-estimates.html
- Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434.
- Centers for Disease Control and Prevention (CDC). National Ambulatory Medical Care Survey. National Center for Health Statistics. Updated December 30, 2021. Accessed Jan, 2024, https://www.cdc.gov/nchs/ahcd/about_ahcd.htm
- Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016;33(3):312-317.
- Centers for Disease Control and Prevention (CDC). Measuring Outpatient Antibiotic Prescribing. Updated Oct 2022. Accessed Jan 2024, https://www.cdc.gov/antibiotic-use/data/outpatient- prescribing/index.html
- Pulingam T, Parumasivam T, Gazzali AM, et al. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci. 2022/03/01/ 2022;170:106103. doi:https://doi.org/10.1016/j.ejps.2021.106103
- Shapiro NI, Self WH, Rosen J, et al. A prospective, multi-centre US clinical trial to determine accuracy of FebriDx point-of-care testing for acute upper respiratory infections with and without a confirmed fever. Ann Med. Aug 2018;50(5):420-429. doi:10.1080/07853890.2018.1474002
- Shapiro NI, Filbin MR, Hou PC, et al. Diagnostic Accuracy of a Bacterial and Viral Biomarker Point-of-Care Test in the Outpatient Setting. JAMA Netw Open. Oct 3 2022;5(10):e2234588.doi:10.1001/jamanetworkopen.2022.34588
The science behind the accuracy

FebriDx = 2 Biomarkers
No single biomarker can differentiate bacterial from non-bacterial infection. The FebriDx test utilizes a proprietary combination of two host immune response biomarkers to aid in differentiating bacterial infection from non-bacterial etiology.1
CRP (C-Reactive Protein) is a nonspecific, acute-phase protein that is upregulated during the presence of acute inflammation, including response to infection. CRP is predominately produced by the liver in response to inflammatory cytokines such as IL-6 and assists in pathogen recognition and phagocytosis by macrophages.2 Infection is a potent stimulus of CRP elevation, which occurs within 4-6 hours of infection, doubles every 8 hours and peaks after 36 hours.3 At low levels CRP is sensitive but non-specific for bacterial infection.4
MxA (Myxovirus resistance protein A) is an innate host response biomarker that elevates in the presence of acute viral infection but is not specific to a particular type of virus. MxA has a low basal concentration of less than 15 ng/mL, a fast induction time of 1-2 hours, and a long half-life of 2.3 days.5 Numerous clinical studies demonstrate that MxA protein expression in peripheral blood has been shown to be a sensitive and specific marker for viral infection.6-7 MxA is specific for viral infection only and is not elevated in the presence of a bacterial infection.6-8
Neither MxA nor CRP alone is sensitive or specific enough to differentiate bacterial infection from non-bacterial etiology.4,6,9,10 At a low-level cut off, 20mg/L, CRP is very sensitive but non-specific at confirming bacterial infection. At a high-level cut off; 80-100mg/L, the reverse is true.3,9
By combining the acute phase inflammatory protein, CRP, with a specific viral marker, MxA, FebriDx achieves the sensitivity and specificity to aid in the differentiation of bacterial from non-bacterial acute respiratory infection.1
LOW-LEVEL CRP CUT OFF MAY LEAD TO THE OVER PRESCRIPTION OF ANTIBIOTICS FOR NON-BACTERIAL ETIOLOGIES.
A HIGH-LEVEL CRP CUT OFF MAY MEAN PATIENTS WITH BACTERIAL INFECTIONS COULD BE MISSED.
FEBRIDX DUAL BIOMARKER TECHNOLOGY (CRP + MXA) CAN DIFFERENTIATE BACTERIAL INFECTION FROM NON-BACTERIAL ETIOLOGIES.
FebriDx multi-center clinical study1
Multi-center, prospective, clinical trial demonstrated high sensitivity and specificity of FebriDx in differentiating bacterial from non-bacterial acute respiratory infection.
The study was conducted at 20 point-of-care testing sites. FebriDx was compared to a composite Clinical Reference Algorithm that incorporated pathogen detection testing including bacterial culture and multiplex PCR in addition to measures of host immune response.
FebriDx has a 99% NPV to rule out a bacterial infection.1
Characteristic |
Estimate |
95% CI |
|---|---|---|
| PPA | 93.2% (68 / 73) | 84.9% - 97.0% |
| NPA | 88.4% (374 / 423) | 85.0% - 91.0% |
| PPV | 58.1% (68 / 117) | 49.1 - 66.7% |
| NPV | 98.7% (374 / 379) | 96.9% - 99.4% |
PPA = positive percent agreement
PPV = positive predictive value
NPA = negative percent agreement
NPV = negative predictive value
- Shapiro NI, Filbin MR, Hou PC, et al. Diagnostic Accuracy of a Bacterial and Viral Biomarker Point-of-Care Test in the Outpatient Setting. JAMA Netw Open. Oct 3 2022;5(10):e2234588. doi:10.1001/jamanetworkopen.2022.34588
- Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunologic Research. 2013/05/01 2013;56(1):131-142. doi:10.1007/s12026-013-8384-0
- Bray C, Bell LN, Liang H, et al. Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine. Wmj. 2016;115(6):317-21.
- Hatherill M, Tibby SM, Sykes K, Turner C, Murdoch IA. Diagnostic markers of infection: comparison of procalcitonin with C reactive protein and leucocyte count. Arch Dis Child. Nov 1999;81(5):417-21. doi:10.1136/adc.81.5.417
- Ronni T, Melén K, Malygin A, Julkunen I. Control of IFN-inducible MxA gene expression in human cells. J Immunol. 1993;150(5):1715-1726.
- Engelmann I, Dubos F, Lobert P-E, et al. Diagnosis of Viral Infections Using Myxovirus Resistance Protein A (MxA). Pediatrics. 2015;135(4):e985-e993. doi:10.1542/peds.2014-1946
- Nakabayashi M, Adachi Y, Itazawa T, et al. MxA-Based Recognition of Viral Illness in Febrile Children by a Whole Blood Assay. Pediatric Research. 2006/12/01 2006;60(6):770-774. doi:10.1203/01.pdr.0000246098.65888.5b
- Piri R, Yahya M, Ivaska L, et al. Myxovirus Resistance Protein A as a Marker of Viral Cause of Illness in Children Hospitalized with an Acute Infection. Microbiol Spectr. Feb 23 2022;10(1):e0203121. doi:10.1128/spectrum.02031-21
- Andreola B, Bressan S, Callegaro S, Liverani A, Plebani M, Da Dalt L. Procalcitonin and C-reactive protein as diagnostic markers of severe bacterial infections in febrile infants and children in the emergency department. Pediatr Infect Dis J. Aug 2007;26(8):672-7. doi:10.1097/INF.0b013e31806215e3
- Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum Procalcitonin and C-Reactive Protein Levels as Markers of Bacterial Infection: A Systematic Review and Meta-analysis. Clin Infect Dis. 2004;39(2):206-217. doi:10.1086/421997
An all-in-one test device
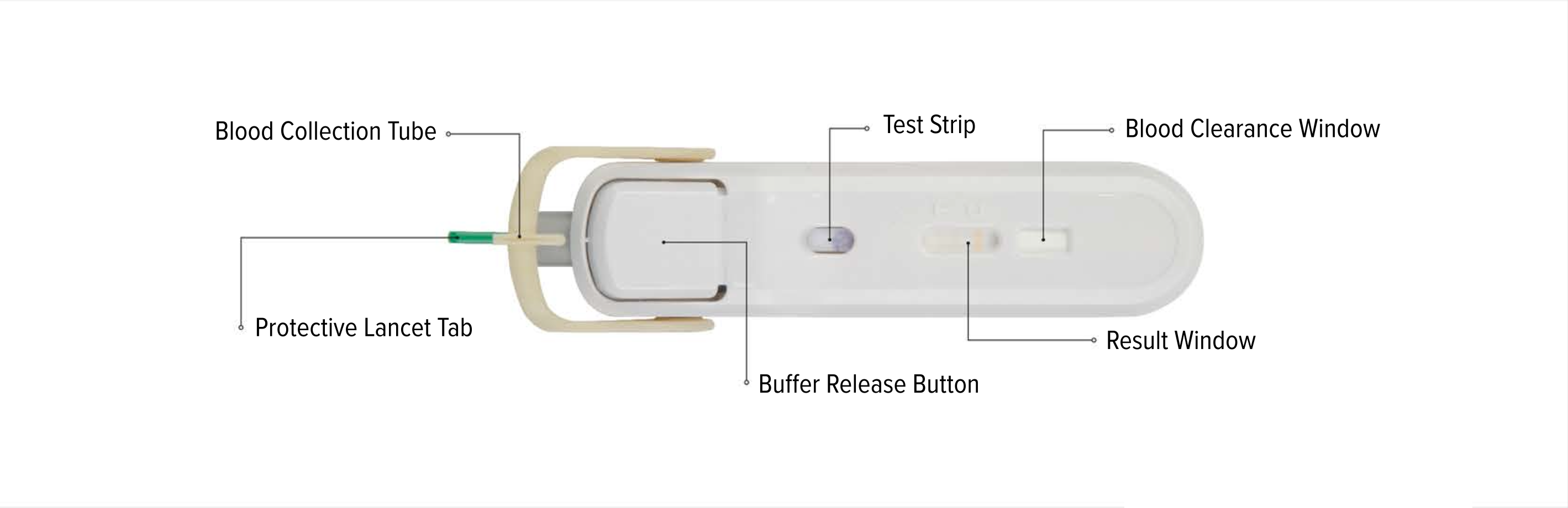
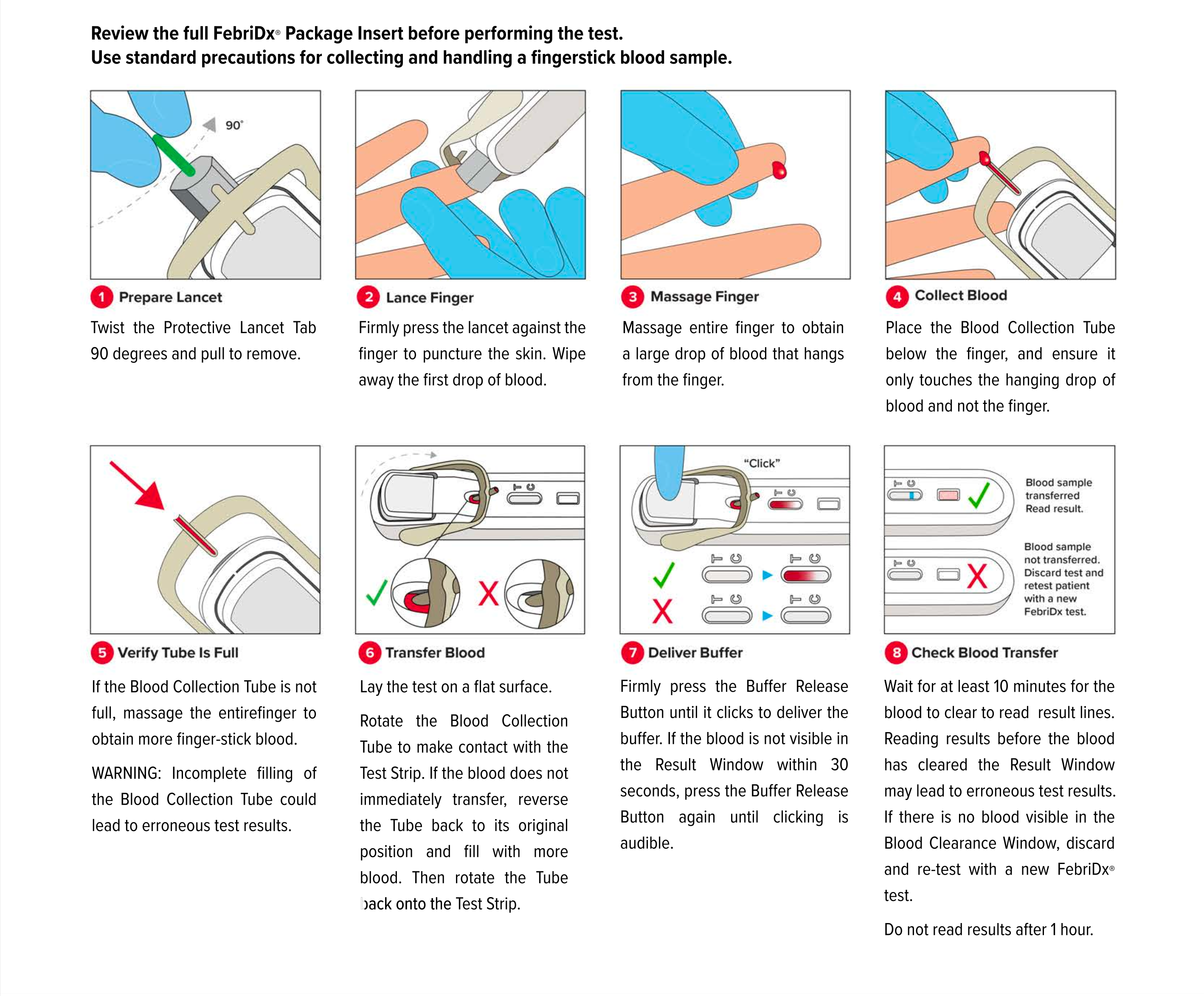
FebriDx instructions for use
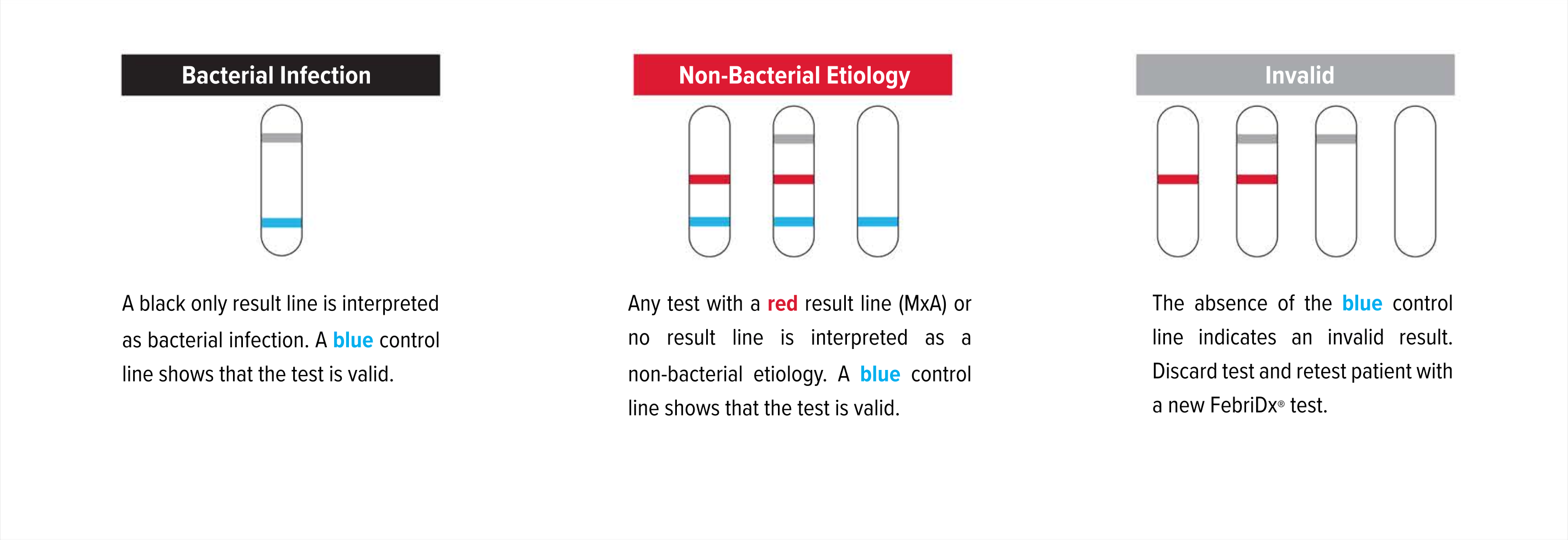
Test Interpretation
FebriDx FAQs
FebriDx tests can be stored at room temperature (4-25°C or 39-77°F).
Patients aged 12-64 who present to urgent care or emergency care settings for evaluation of acute respiratory infection who have had symptoms for less than 7 days and within 3 days of fever onset.
Only fresh fingerstick blood can be used with the FebriDx test. Venous whole blood cannot be used.
FebriDx can be read after 10 minutes has elapsed and the background is clear of blood. FebriDx test results are stable for up to one hour. Do not attempt to interpret results beyond one hour of test initiation.
Even if the result line is faint in color, incomplete over the width of the test strip, or uneven in color, it should be interpreted as positive. A positive result indicates the presence of elevated MxA and/or CRP proteins.
A blue control line must appear in the result window for the test to be valid. The absence of the blue control line indicates an invalid result and the patient must be retested with a new FebriDx test.
FebriDx |
||
|---|---|---|
Product Name |
Part number |
Kit size |
| FebriDx Bacterial / Non-Bacterial Point-of-Care Assay | CP0014 | 25 tests |
| FebriDx External Controls | CP0007 | 1 positive control, 1 negative control, 44 pipettes (2 bags x 22 pipettes) |
